A convenient route to internally phosphane-stabilized aryltriborane(7) compounds†
Abstract
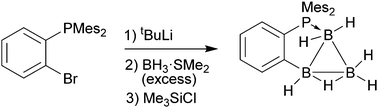
The biphenylene derived triborane(7) derivatives 7 featuring an internal phosphane donor group can readily be prepared from the respective in situ generated aryllithium compound with excess BH3·SMe2. The internal donor group assisted assembly procedure was also applied to the synthesis of the phenylene bonded triborane(7) compound 11. These aryl-triborane(7) systems can further react with HB(C6F5)2 to give the doubly C6F5 functionalized derivatives 12 and 13. The products were characterized spectroscopically and by X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...