Asymmetric total synthesis of pleurospiroketals A and B†
Abstract
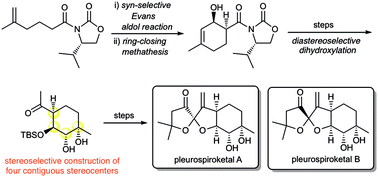
The first asymmetric total synthesis of pleurospiroketals A and B has been accomplished in 16 steps from 5-methyl-5-hexenoic acid. Key features of the synthesis are the highly syn-selective Evans aldol reaction, ring-closing metathesis, highly diastereoselective dihydroxylation and acid-mediated spiroketalization.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...