Direct α-C–H amination using various amino agents by selective oxidative copper catalysis: a divergent access to functional quinolines†
Abstract
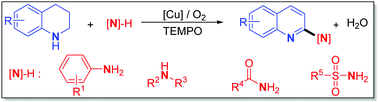
Herein, we present, for the first time, direct dehydrogenative α-C–H amination of tetrahydroquinolines (THQs) using various amino agents by selective aerobic copper catalysis, which enables divergent access to 2-aminoquinolines, the core structures of numerous functional products. In which, the catalyst system preferentially oxidizes the tetrahydroquinolines between two amino reactants, and the presence of TEMPO significantly enhances the capability of the first oxidation of THQs and makes it a kinetically controlled process, thus favoring the C–N bond-forming step. The developed chemistry features broad substrates, excellent functional tolerance, high chemo-selectivity, and no need for pre-preparation of specific aminating agents, which offers a practical way for diverse and atom-economic synthesis of 2-aminoquinolines that are difficult to prepare or inaccessible with the existing C–H amination approaches.


 Please wait while we load your content...
Please wait while we load your content...