Sydnone-coumarins as clickable turn-on fluorescent sensors for molecular imaging†
Abstract
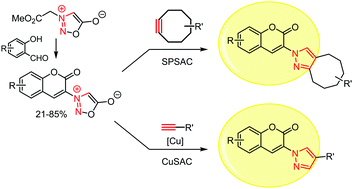
Copper-catalyzed and copper-free sydnone–alkyne cycloaddition reactions have emerged as complementary click tools for chemical biology but their use in bioorthogonal labeling is still in its infancy. Herein, combinations of alkynes and coumarin-sydnones were screened for their ability to generate pyrazole products displaying strong fluoroscence enhancement compared to reactants. One sydnone was identified as a particularly suitable new turn-on probe for protein labeling.


 Please wait while we load your content...
Please wait while we load your content...