Palladium-catalyzed sequential three-component reactions to access vinylsilanes†
Abstract
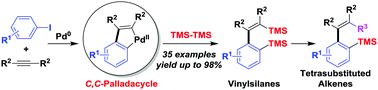
A palladium-catalyzed sequential three-component reaction has been developed. The palladacycles, generated through cascade reactions of aryl halides and alkynes, are the key intermediates and react with hexamethyldisilane to form disilylated products. The reaction represents a useful preparative method for vinylsilanes, and the vinylsilanes can be transformed into tetrasubstituted alkenes.


 Please wait while we load your content...
Please wait while we load your content...