Essential but sparse collagen hydroxylysyl post-translational modifications detected by DNP NMR†
Abstract
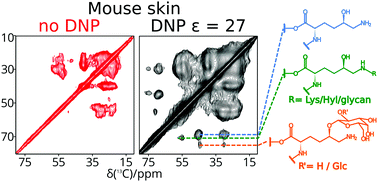
The sparse but functionally essential post-translational collagen modification 5-hydroxylysine can undergo further transformations, including crosslinking, O-glycosylation, and glycation. Dynamic nuclear polarization (DNP) and stable isotope enriched lysine incorporation provide sufficient solid-state NMR sensitivity to identify these adducts directly in skin and vascular smooth muscle cell extracellular matrix (ECM), without extraction procedures, by comparison with chemical shifts of model compounds. Thus, DNP provides access to the elucidation of structural consequences of collagen modifications in intact tissue.


 Please wait while we load your content...
Please wait while we load your content...