Real-time tracking of single-molecule collagenase on native collagen and partially structured collagen-mimic substrates†
Abstract
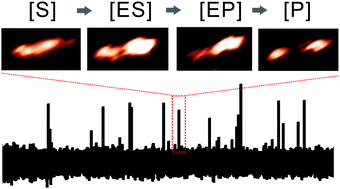
The dynamic interactions of an individual matrix metalloproteinase-1 were imaged and monitored in the presence of either triple-helical or non-triple-helical, partially structured collagen-mimic substrates. The enzyme exhibited ten-fold increased catalytic turnover rates with the structurally modified substrate by skipping the triple-helix unwinding step during the catalytic pathway.


 Please wait while we load your content...
Please wait while we load your content...