1,2-Diarylation of alkenes with aryldiazonium salts and arenes enabled by visible light photoredox catalysis†
Abstract
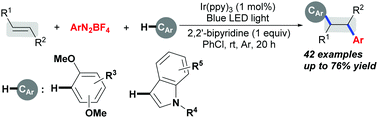
A mild and general visible light photoredox catalysis-induced intermolecular three-component alkene 1,2-diarylation involving aryl C(sp2)–H functionalization is described. The key to controlling the chemoselectivity toward alkene 1,2-diarylation is the employment of a 2,2′-bipyridine base, thus allowing the formation of two new C(sp3)–C(sp2) bonds via aryl radical formation from aryldiazonium salts, addition across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, and aryl C(sp2)–H functionalization cascades.
C bonds, and aryl C(sp2)–H functionalization cascades.


 Please wait while we load your content...
Please wait while we load your content...