Copper(i)-catalyzed N–H olefination of sulfonamides for N-sulfonyl enaminone synthesis†
Abstract
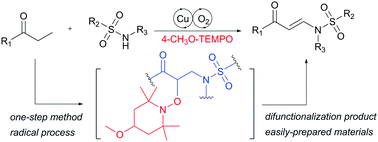
This communication reports copper-catalyzed N–H olefination of sulfonamides for enaminone synthesis using saturated ketones as olefin sources. With TEMPO derivatives and O2 as oxidants, this method provided an efficient way to produce various enaminones in good yields. Mechanistic studies helped figure out the stable intermediates and develop novel methodologies for the difunctionalization of saturated ketones.


 Please wait while we load your content...
Please wait while we load your content...