Controlled hydroxy-fluorination reaction of anatase to promote Mg2+ mobility in rechargeable magnesium batteries†‡
Abstract
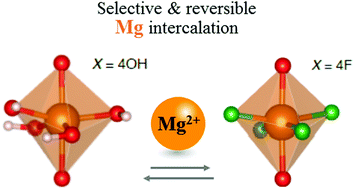
In anatase TiO2, substituting oxide anions with singly charged (F,OH) anions allows the controlled formation of cation vacancies, which act as reversible intercalation sites for Mg2+. We show that ion-transport (diffusion coefficients) and intercalation (reversible capacity) properties are controlled by two critical parameters: the vacancy concentration and the local anionic environment. Our results emphasise the complexity of this behaviour, and highlight the potential benefits of chemically controlling cationic-defects in electrode materials for rechargeable multivalent-ion batteries.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...