Diastereoselective addition of anisoles to N-tert-butanesulfinyl imines via four-membered lithium cycles†
Abstract
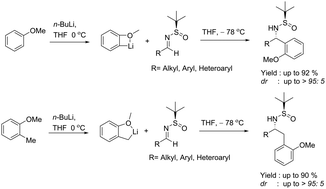
A highly regio- and diastereo-selective ortho-lithiation/addition of anisoles to N-tert-butanesulfinyl imines resulting in the selective formation of chiral α-branched amines is described. This method is also efficient for highly regioselective benzylic lithiation of o-methylanisoles, followed by diastereoselective addition to N-tert-butanesulfinyl imines.


 Please wait while we load your content...
Please wait while we load your content...