Synthesis of α-CF3 and α-CF2H amines via the aminofluorination of fluorinated alkenes†
Abstract
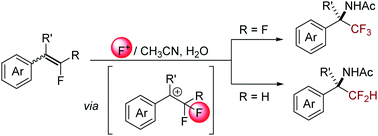
A novel synthesis of α-CF3 and α-CF2H amines via the aminofluorination of gem-difluoroalkenes and mono-fluoroalkenes, respectively, is reported. The method employs Selectfluor as an electrophilic fluorine source and acetonitrile as a nitrogen source. Mechanistic studies revealed a single-electron oxidation/fluorine-abstraction/Ritter-type amination pathway. The protocol allowed the synthesis of a broad range of fluorinated amines including those bearing quaternary carbon centers with good efficiency and functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...