Synthesis of zero-valent iron nanoparticles via laser ablation in a formate ionic liquid under atmospheric conditions†
Abstract
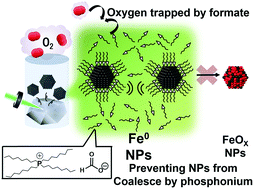
Transition metal nanoparticles (NPs) are promising materials for use as catalysts in many processes, although they are easily oxidized under ambient conditions. In this communication, a novel synthetic method is proposed for producing zero-valent iron (Fe) NPs by laser ablation under atmospheric conditions using the reducing properties of a formate-based ionic liquid solvent. The valence state of Fe was confirmed using X-ray absorption near edge structure (XANES) spectroscopy. The Fe NPs adopt a face centered cubic structure after synthesis, which gradually transforms to a body centered cubic structure after one month. The method can be extended to the synthesis of other transition metal NPs that are easily oxidized.


 Please wait while we load your content...
Please wait while we load your content...