Design of functionalized cyclic peptides through orthogonal click reactions for cell culture and targeting applications†
Abstract
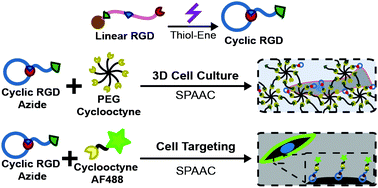
An approach for the design of functionalized cyclic peptides is established for use in 3D cell culture and in cell targeting. Sequential orthogonal click reactions, specifically a photoinitiated thiol–ene and strain promoted azide–alkyne cycloaddition, were utilized for peptide cyclization and conjugation relevant for biomaterial and biomedical applications, respectively.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...