Fluorescence activation with switchable oxazines
Abstract
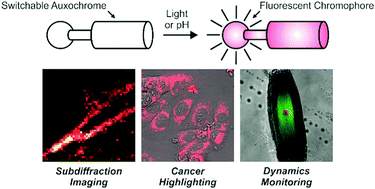
The identification of operating principles to activate fluorescence under the influence of external stimulations is essential to enable the implementation of imaging strategies requiring the spatiotemporal control of emission. In this context, our laboratories designed mechanisms to switch fluorescence with either light or pH based on the unique photochemical and photophysical properties of either photoresponsive or halochromic oxazines respectively. These heterocycles can be connected covalently to fluorescent chromophores and opened with either light or pH to impose a significant bathochromic shift on the main absorption of the emissive appendage. Such a spectral change allows the selective excitation of the resulting species to activate bright fluorescence with infinite contrast and spatiotemporal control. Indeed, these mechanisms for fluorescence activation enable the acquisition of images with subdiffraction resolution, the selective signaling of cancer cells and the monitoring of translocating species in real time. Thus, our structural designs for fluorescence switching under external control can evolve into invaluable probes for the implementation of bioimaging strategies that would be impossible to perform with conventional fluorophores.


 Please wait while we load your content...
Please wait while we load your content...