A planarized B-phenyldibenzoborepin: impact of structural constraint on its electronic properties and Lewis acidity†
Abstract
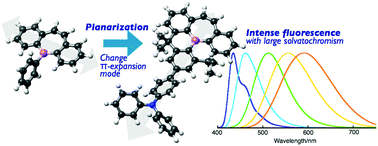
A B-phenyldibenzo[b,f]borepin planarized with two methylene bridges was synthesized. The structural constraint on the B-phenyl group resulted in a bathochromic shift of the absorption and fluorescence properties as well as enhanced Lewis acidity. A donor–π–acceptor type derivative based on this scaffold exhibited intense fluorescence irrespective of the solvent polarity.


 Please wait while we load your content...
Please wait while we load your content...