Dimerization of boryl- and amino-substituted acetylenes by B2C2 four-membered ring formation†
Abstract
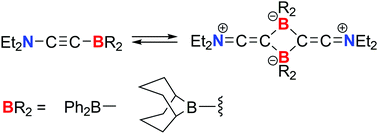
Boryl- and amino-substituted acetylenes bearing diphenylboryl or 9-borabicyclononyl groups were synthesized. X-ray diffraction analyses revealed the dimerized structures of these acetylenes via the formation of B2C2 four-membered rings. Spectroscopic studies and DFT calculations indicated that these dimers can dissociate to afford monomeric acetylenes, and that the equilibrium constant for the dissociation depends on the structure of the boryl substituents.


 Please wait while we load your content...
Please wait while we load your content...