Manganese catalyzed reductive amination of aldehydes using hydrogen as a reductant†
Abstract
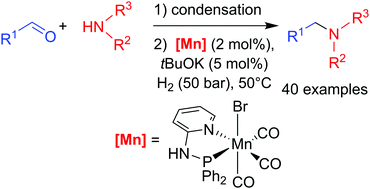
A one-pot two-step procedure was developed for the alkylation of amines via reductive amination of aldehydes using molecular dihydrogen as a reductant in the presence of a manganese pyridinyl-phosphine complex as a pre-catalyst. After the initial condensation step, the reduction of imines formed in situ is performed under mild conditions (50–100 °C) with 2 mol% of catalyst and 5 mol% of tBuOK under 50 bar of hydrogen. Excellent yields (>90%) were obtained for a large combination of aldehydes and amines (40 examples), including aliphatic aldehydes and amino-alcohols.


 Please wait while we load your content...
Please wait while we load your content...