Aggregation kinetics of the Aβ1–40 peptide monitored by NMR†
Abstract
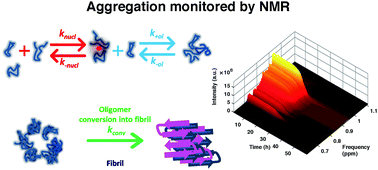
The aggregation of Aβ1–40 was monitored by solution NMR, which showed a trend complementary to the one observed by ThT-fluorescence. The NMR data support a kinetic model where Aβ1–40 initially aggregates with the reversible formation of oligomeric species, which then irreversibly convert into fibrils.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...