Palladium-catalyzed aerobic regio- and stereo-selective olefination reactions of phenols and acrylates via direct dehydrogenative C(sp2)–O cross-coupling†
Abstract
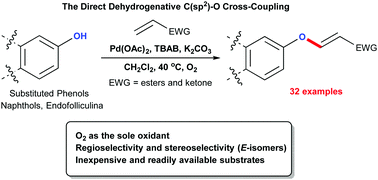
An efficient olefination protocol for the oxidative dehydrogenation of phenols and acrylates has been achieved using a palladium catalyst and O2 as the sole oxidant. This reaction exhibits high regio- and stereo-selectivity (E-isomers) with moderate to excellent isolated yields and a wide substrate scope (32 examples) including ethyl vinyl ketone and endofolliculina.


 Please wait while we load your content...
Please wait while we load your content...