A chiral organic base catalyst with halogen-bonding-donor functionality: asymmetric Mannich reactions of malononitrile with N-Boc aldimines and ketimines†
Abstract
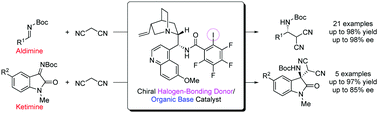
A chiral organic base catalyst with halogen-bonding-donor functionality has been developed. This quinidine-derived acid/base catalyst smoothly promoted the asymmetric Mannich reaction of malononitrile and various N-Boc imines with up to 98% ee. The cooperative interaction with both substrates was responsible for the high activity that allowed a reduction of the catalyst amount to 0.5 mol%.


 Please wait while we load your content...
Please wait while we load your content...