Catalytic, metal-free sulfonylcyanation of alkenes via visible light organophotoredox catalysis†
Abstract
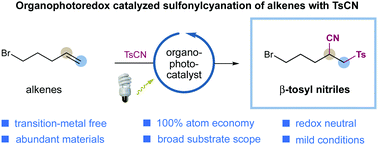
The group transfer radical addition of olefins with tosyl cyanide has been accomplished via visible light-induced organophotoredox catalysis. A diverse array of olefins is amenable to this protocol, furnishing β-sulfonyl nitriles with excellent efficiency under metal-free and redox-neutral conditions. A closed catalytic cycle is operative in this transformation, providing complementary reactivity to the classic radical chain process.


 Please wait while we load your content...
Please wait while we load your content...