2-Oxoglutarate regulates binding of hydroxylated hypoxia-inducible factor to prolyl hydroxylase domain 2†
Abstract
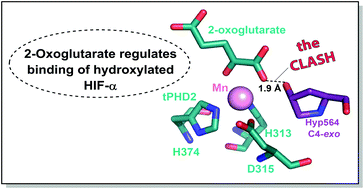
Prolyl hydroxylation of hypoxia inducible factor (HIF)-α, as catalysed by the Fe(II)/2-oxoglutarate (2OG)-dependent prolyl hydroxylase domain (PHD) enzymes, has a hypoxia sensing role in animals. We report that binding of prolyl-hydroxylated HIF-α to PHD2 is ∼50 fold hindered by prior 2OG binding; thus, when 2OG is limiting, HIF-α degradation might be inhibited by PHD binding.


 Please wait while we load your content...
Please wait while we load your content...