Enzymatically-stable oxetane-based dipeptide hydrogels†
Abstract
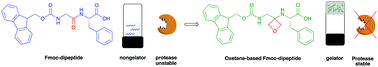
Low molecular weight gelators that are not easily degraded by enzymes have a range of potential applications. Here, we report new Fmoc-protected dipeptides in which the amide carbonyl group has been replaced by an oxetane ring. Remarkably one of these peptidomimetics, but not the corresponding dipeptide, is an effective gelator, forming hydrogels at a concentration of 3 mg mL−1. On assembly, there is a lack of beta-sheet structure, implying that there is no requirement for this motif in such a gel. Furthermore, the modified dipeptide is also stable to proteolysis compared to the parent dipeptide.


 Please wait while we load your content...
Please wait while we load your content...