Dynamical heterogeneities in ionic liquids as revealed from deuteron NMR†
Abstract
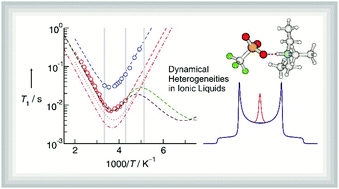
The heterogeneity in dynamics has important consequences for understanding the viscosity, diffusion, ionic mobility, and the rates of chemical reactions in technology relevant systems such as polymers, metallic glasses, aqueous solutions, and inorganic materials. Herein, we study the spatial and dynamic heterogeneities in ionic liquids by means of solid state NMR spectroscopy. In the 2H spectra of the protic ionic liquid [TEA][OTf] we observe anisotropic and isotropic signals at the same time. The spectra measured below the melting temperature at 306 K could be simulated by a superposition of the solid and liquid line shapes, which provided the transition enthalpies between the rigid and mobile fractions. Consequently, we measured the spin–lattice relaxation times T1 for the anisotropic and the isotropic signals for the temperature range between 203 and 436 K. Both dispersion curves could be fitted to models including rotational correlation times, activation barriers and rate constants. This approach allowed determining the rotational correlation times for the N–D molecular vector of the [TEA]+ cation in differently mobile environments. The mobility is only slightly different, as indicated by small differences in activation energies for these processes. The NMR correlation times for the highly mobile phase are linearly related to measured viscosities, which supports the applicability of the Stokes–Einstein–Debye relation.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...