Oxidative organocatalytic chemoselective N-acylation of heterocycles with aromatic and conjugated aldehydes†
Abstract
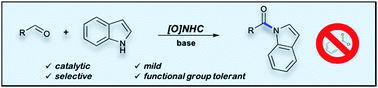
Selective acylation of indoles is cumbersome often involving the need for sensitive and reactive acyl chloride derivatives or coupling reagents. Here we report a mild, functional group tolerant and highly chemoselective oxidative carbene catalyzed N-acylation of indoles with aldehydes. The acylation has a broad substrate scope and is compatible with substituents on both the aldehyde and the indole reaction partner. Furthermore, aza-heterocycles such as pyrrole and indazole can also be used as nucleophiles in this reaction providing the corresponding amide congeners in good yield.


 Please wait while we load your content...
Please wait while we load your content...