Electrocatalytic CO2 reduction by a cobalt bis(pyridylmonoimine) complex: effect of acid concentration on catalyst activity and stability†
Abstract
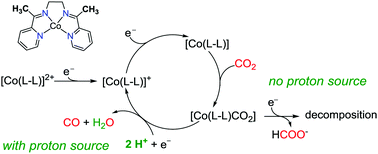
A Co complex with a redox-active bis(pyridylmonoimine) ligand has been prepared and shows catalytic activity for electrochemical CO2 reduction in acetonitrile. Addition of a proton source such as water or trifluoroethanol dramatically improves the activity and stability of the molecular catalyst. The Co complex reduces CO2 to CO selectively at −1.95 V vs. Fc+/0 in the presence of high concentrations of water. The activity of the Co complex for CO2 reduction compares favorably to other molecular Co-based catalysts in acetonitrile solutions.


 Please wait while we load your content...
Please wait while we load your content...