Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols†
Abstract
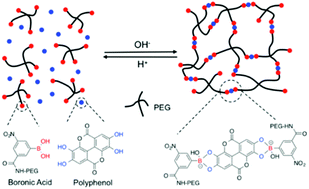
We report here the development of hydrogels formed at physiological conditions using PEG (polyethylene glycol) based polymers modified with boronic acids (BAs) as backbones and the plant derived polyphenols ellagic acid (EA), epigallocatechin gallate (EGCG), tannic acid (TA), nordihydroguaiaretic acid (NDGA), rutin trihydrate (RT), rosmarinic acid (RA) and carminic acid (CA) as linkers. Rheological frequency sweep and single molecule force spectroscopy (SMFS) experiments show that hydrogels linked with EGCG and TA are mechanically stiff, arising from the dynamic covalent bond formed by the polyphenol linker and boronic acid functionalized polymer. Stability tests of the hydrogels in physiological conditions revealed that gels linked with EA, EGCG, and TA are stable. We furthermore showed that EA- and EGCG-linked hydrogels can be formed via in situ gelation in pH 7.4 buffer, and provide long-term steady state release of bioactive EA. In vitro experiments showed that EA-linked hydrogel significantly reduced the viability of CAL-27 human oral cancer cells via gradual release of EA.
- This article is part of the themed collection: Biomaterials Science 10th Anniversary: Top papers from North America


 Please wait while we load your content...
Please wait while we load your content...