A silencing-mediated enhancement of osteogenic differentiation by supramolecular ternary siRNA polyplexes comprising biocleavable cationic polyrotaxanes and anionic fusogenic peptides†
Abstract
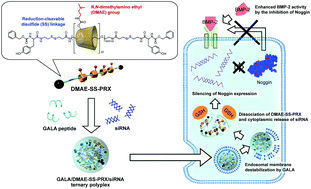
Gene silencing of noggin by small interfering RNA (siRNA) is a promising approach for the treatment of bone defects, because noggin deactivates bone morphogenetic protein-2 (BMP-2) and suppresses osteogenic differentiation. Here, we demonstrated the silencing of the noggin gene by siRNA polyplexes composed of noggin-targeted siRNA and biocleavable cationic polyrotaxanes (DMAE-SS-PRX). To improve the endosomal escape efficiencies of the DMAE-SS-PRX/siRNA polyplexes, anionic and fusogenic GALA peptides were integrated onto the DMAE-SS-PRX/siRNA polyplexes via simple electrostatic interactions. The formation of ternary complexes was confirmed by gel electrophoresis, dynamic light scattering, and zeta-potential measurements. Although the association of GALA peptides with the DMAE-SS-PRX/siRNA polyplexes did not remarkably affect the cellular uptake efficiency of siRNA, the endosomal escape efficiency was remarkably increased for GALA/DMAE-SS-PRX/siRNA ternary polyplexes because of the endosomal and lysosomal membrane destabilization by GALA peptides. Consequently, GALA/DMAE-SS-PRX/siRNA ternary polyplexes showed significantly higher gene silencing efficiency against noggin and enhanced the BMP-2-mediated osteogenic differentiation efficiency. Therefore, we concluded that GALA/DMAE-SS-PRX/siRNA ternary polyplexes can be effective siRNA carriers for suppressing the expression of specific endogenous genes. Consequently, we believe that a more practical approach in vivo will be the combined use of BMP-2 and GALA/DMAE-SS-PRX/siRNA ternary polyplexes, because it will improve the efficacy of bone regeneration therapy.


 Please wait while we load your content...
Please wait while we load your content...