Development and validation of a robust analytical method to quantify both etoposide and prodigiosin in polymeric nanoparticles by reverse-phase high-performance liquid chromatography
Abstract
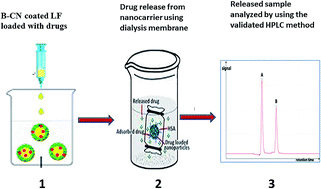
The objective of this work was to develop and validate a rapid reversed-phase high-performance liquid chromatography method for the quantification of etoposide (ETP) and prodigiosin (PRO) content in a nanoparticle delivery system prepared with β-casein coated lactoferrin for targeted delivery to lung cancer cells. Chromatographic runs were performed on a Shimadzu Prominence LC-20AP with an RP Prontosil Kromaplus phenyl (250 × 4.6 mm i.d., 5 μm) column using methanol and 10 mM ammonium acetate buffer (75 : 25 v/v) as a mobile phase in an isocratic elution, with a fixed pH of 3.0 ± 0.2 at a flow rate of 2 ml min−1. ETP was detected at wavelengths of 242 and 286 nm, while PRO was estimated at a wavelength of 536 nm. The injection volume was 20 μl and the column temperature was maintained at ambient temperature (25 °C ± 0.5). The validation characteristics included accuracy, precision, specificity, linearity, recovery, and robustness. The standard curve was found to have a linear relationship (r = 0.99957 for ETP242, 0.99997 for ETP286 and 0.99996 for PRO536) over the analytical range of 1–100 μg ml−1. The detection and quantitation limits were 0.30 and 0.99 μg ml−1 for ETP242, 0.45 and 1.50 μg ml−1 for ETP286 and 0.37 and 0.74 μg ml−1 for PRO536, respectively. The recovery and loaded ETP and PRO in the nano-micelle delivery system were nearly 100% and 98%, respectively. The present data greatly underpin a new, robust, fast, precise, validated HPLC based method for the quantification of ETP and PRO in nanoformulations.


 Please wait while we load your content...
Please wait while we load your content...