The doping level of boron-doped diamond electrodes affects the voltammetric sensing of uric acid
Abstract
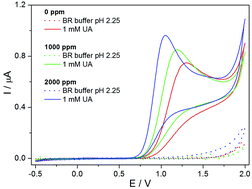
In this work, the electrochemical oxidation and subsequent determination of uric acid was investigated using boron-doped diamond electrodes with various B/C ratios (0–2000 ppm). The cyclic voltammetric study showed one irreversible oxidation peak at +(1.1–1.25) V (vs. Ag/AgCl/3 M KCl) in the presence of Britton–Robinson buffer (pH 2.25) depending on the boron content. Employing differential pulse voltammetry using the 2000 ppm boron-doped diamond electrode the acquired analytical parameters were as follows: a limit of detection of 7.7 μM, a limit of quantification of 26 μM and intra-day repeatability (relative standard deviation of 2.9% for n = 15). After performing an interference study, the method was applied to the determination of uric acid in biological samples (human urine). The uric acid concentrations determined in the urine samples were compared with the reference values stated in the literature. The proposed methodology using boron-doped diamond electrodes could find applications in uric acid sensing within clinical, pharmaceutical and environmental analysis.


 Please wait while we load your content...
Please wait while we load your content...