A voltammetric method for Fe(iii) in blood serum using a screen-printed electrode modified with a Schiff base ionophore†
Abstract
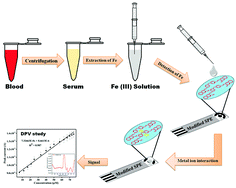
Herein, a potent electrochemical ionophore (SMS-2) based on a Schiff base has been used for the modification of a screen-printed electrode (SPE). The modified disposable electrode can selectively detect ferric ions in an aqueous medium. Redox behavior of the proposed strip was characterized using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Incorporation of the ligand in the ink of the SPE enhanced the analytical performance of the electrode, and its surface modification was confirmed by SEM and EDX analysis. Shifting/quenching of the cathodic peak potential of the ionophore after binding with Fe(III) ions was used to detect and measure the ferric ion concentration. This sensor can identify Fe(III) in the detection range from 0.625 μM to 7.5 μM. The modified SPE can selectively detect ferric ions in the presence of many other interfering ions and has been successfully used to determine the Fe(III) content in blood serum samples. The metal–ionophore complex structure was optimized using DFT calculations to study the energetics of the metal–ionophore interactions.


 Please wait while we load your content...
Please wait while we load your content...