Metal ions induced secondary structure rearrangements: mechanically interlocked lasso vs. unthreaded branched-cyclic topoisomers†
Abstract
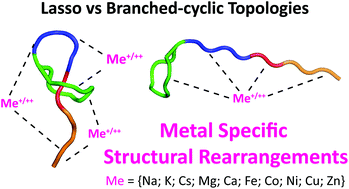
Metal ions can play a significant role in a variety of important functions in protein systems including cofactor for catalysis, protein folding, assembly, structural stability and conformational change. In the present work, we examined the influence of alkali (Na, K and Cs), alkaline earth (Mg and Ca) and transition (Co, Ni and Zn) metal ions on the conformational space and analytical separation of mechanically interlocked lasso peptides. Syanodin I, sphingonodin I, caulonodin III and microcin J25, selected as models of lasso peptides, and their respective branched-cyclic topoisomers were submitted to native nESI trapped ion mobility spectrometry-mass spectrometry (TIMS-MS). The high mobility resolving power of TIMS permitted to group conformational families regardless of the metal ion. The lower diversity of conformational families for syanodin I as compared to the other lasso peptides supports that syanodin I probably forms tighter binding interactions with metal ions limiting their conformational space in the gas-phase. Conversely, the higher diversity of conformational families for the branched-cyclic topologies further supports that the metal ions probably interact with a higher number of electronegative groups arising from the fully unconstraint C-terminal part. A correlation between the lengths of the loop and the C-terminal tail with the conformational space of lasso peptides becomes apparent upon addition of metal ions. It was shown that the threaded C-terminal region in lasso peptides allows only for distinct interactions of the metal ion with either residues in the loop or tail region. This limits the size of the interacting region and apparently leads to a bias of metal ion binding in either the loop or tail region, depending whichever section is larger in the respective lasso peptide. For branched-cyclic peptides, the non-restricted C-terminal tail allows metal coordination by residues throughout this region, which can result in gas-phase structures that are sometimes even more compact than the lasso peptides. The high TIMS resolution also resulted in the separation of almost all lasso and branched-cyclic topoisomer metal ions (r ∼ 2.1 on average). It is also shown that the metal incorporation (e.g., doubly cesiated species) can lead to the formation of a simplified IMS pattern (or preferential conformers), which results in baseline analytical separation and discrimination between lasso and branched-cyclic topologies using TIMS-MS.


 Please wait while we load your content...
Please wait while we load your content...