Noncompetitive homogeneous immunodetection of small molecules based on beta-glucuronidase complementation†
Abstract
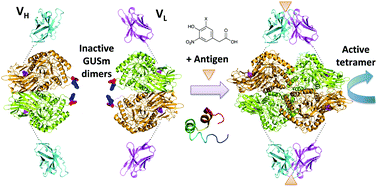
In this study, a novel noncompetitive homogeneous immunoassay for antigen detection was developed. We utilized β-glucuronidase (GUS), a homotetrameric enzyme, the assembly of all of whose subunits is necessary to attain its activity. By using a mutant GUS (GUSm), wherein the dimerization of dimers, which is a rate-limiting step, can be effectively inhibited by a set of interface mutations, we attempted to create a biosensor for detecting various molecules. Usually, the affinity between the two variable region domains (VH and VL) of an antibody, especially for a small molecule, is relatively low. However, in the presence of an antigen, the affinity increases so that they bind tighter to each other. A pair of fusion proteins, comprising the VH and VL regions of the antibody as the detector tethered to a GUSm subunit as the reporter, was constructed to detect antigen 4-hydroxy-3-nitrophenylacetyl (NP) and bone Gla protein (BGP) through GUS activity measurement. Colorimetric and fluorescence assays could detect NP, 5-iodo-NP, and BGP within 1 h without separation steps and with a higher signal/background ratio than conventional ELISA. The instantaneous response after simple mixing of the components makes this system convenient and high-throughput. The system could be effective for the analyses of various small molecules in environmental and clinical settings.

 Please wait while we load your content...
Please wait while we load your content...