Synthesis, crystal structure, magnetic and electronic properties of the caesium-based transition metal halide Cs3Fe2Br9†
Abstract
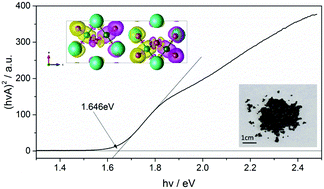
The diversity of halide materials related to important solar energy systems such as CsPbX3 (X = Cl, Br, I) is explored by introducing the transition metal element Fe. In particular a new compound, Cs3Fe2Br9 (space group P63/mmc with a = 7.5427(8) and c = 18.5849(13) Å), has been synthesized and found to contain 0D face-sharing Fe2Br9 octahedral dimers. Unlike its isomorph, Cs3Bi2I9, it is black in color, has a low optical bandgap of 1.65 eV and exhibits antiferromagnetic behavior below TN = 13 K. Density functional theory calculations shed further light on these properties and also predict that the material should have anisotropic transport characteristics.
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...