Manipulating organic triplet harvesting in regioisomeric microcrystals†
Abstract
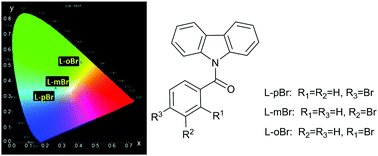
A big step towards manipulating organic triplet harvesting has been taken in three N-benzoyl-carbazole regioisomeric microcrystals (L-(p,m,o)Br) which consist of a –Br group at the para (p)/meta (m)/ortho (o) positions of the phenyl ring, respectively. The manipulation is demonstrated by blue thermally activated delayed fluorescence (TADF) emission in L-pBr, blue and yellow dual room temperature phosphorescence (RTP) emissions with different lifetimes in L-mBr, and ultralong yellow RTP in L-oBr. The effect of the –Br group was verified in molecular excited electronic structures and packing modes upon crystallization. Single crystal analysis and theoretical calculations reveal that exciton structures in the L-(p,m,o)Br dimers are strongly influenced by the bromine substitution positions, leading to different ways to harvest triplet excitons. As a simple and feasible strategy, taking advantage of the substitution position effect is promising in the manipulation of organic triplet excitons to achieve multifarious applications based on triplet exciton properties.


 Please wait while we load your content...
Please wait while we load your content...