Insight into the interactions between pyrene and polystyrene for efficient quenching nitroaromatic explosives†
Abstract
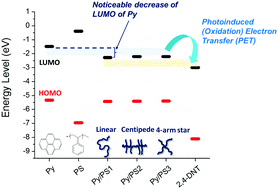
A pyrene (Py) in polystyrene (PS) matrix shows rapid fluorescence quenching in the presence of 2,4-dinitrotoluene (2,4-DNT). The fluorescence quenching does not occur in the absence of the PS matrix, implying that the molecular architecture of the PS chain is critical. However, the function of the PS matrix is not well understood. Here, we investigate various Py/PS binary thin films containing PS of diverse molecular architecture (i.e., linear, centipede and 4-arm star) and molecular weight (i.e., 2.5, 35, 192, 350 and 900 kDa) to understand the effects of these molecular descriptors on the fluorescence quenching. The findings suggest that the electron-rich nature of Py/PS facilitates the photoinduced electron transfer from Py/PS to the electron-deficient 2,4-DNT, resulting in effective quenching of Py excimers. Moreover, cyclic voltammetry (CV) and UV-vis absorption verified that PS reduces the lowest unoccupied molecular orbital level of Py, promoting the Py excimer quenching efficiency in the presence of nitroaromatic molecules. The quenching process is found to be independent of molecular architecture and molecular weight, suggesting that energy migration along the PS backbone may not be the key mechanism. This simple and concise concept provide the insight into the selection for highly-efficient sensing materials.


 Please wait while we load your content...
Please wait while we load your content...