Energy transfer assisted solvent effects on CsPbBr3 quantum dots†
Abstract
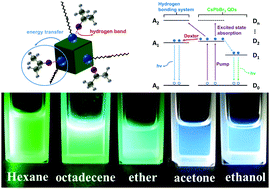
In this work, the solvent effects of chemically synthesized CsPbBr3 quantum dots (QDs) were investigated by dissolving the QDs into solvents of different polarities. The QDs exhibited green fluorescence in non-polar solvents like 1-octadecene, hexane and ether, but exhibited blue fluorescent color in solvents of higher polarities (ethanol or acetone). The photoluminescence (PL), Fourier transform infrared (FTIR), transmission electron microscope (TEM) and time-resolved PL characterization revealed the difference between QDs in the two types of solvents. The hydrogen bonding system of –NH2 and polar solvents was responsible for the different fluorescent colors. Also, energy transfer between the QDs and the hydrogen bonding system was observed and confirmed by using ultrafast femtosecond transient absorption spectroscopy. We expect that the solvent effects of CsPbBr3 QDs have potential applications in fluorescence probes and photoelectrical devices.


 Please wait while we load your content...
Please wait while we load your content...