Synthesis of an aggregation-induced emission (AIE) active salicylaldehyde based Schiff base: study of mechanoluminescence and sensitive Zn(ii) sensing†
Abstract
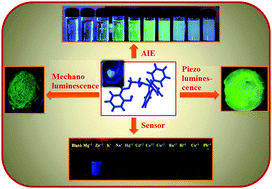
Syntheses of multi-functional Aggregation-Induced Emission (AIE) active molecules in a simple manner have been drawing great attention in current luminescence materials research. In this report a simple diamine molecule (N1-tritylethane-1,2-diamine(1)) is reacted with salicylaldehyde using a Schiff-base technique which results in a new AIE active organic molecule [2-((2-(tritylamino)ethylideneamino)methyl)phenol (2)]. Computational calculations support that the nature of the transition is intra-molecular charge transfer/twisted intramolecular charge transfer (ICT/TICT). The mechanism of AIE has been attributed to restricted intramolecular rotation (RIR). Packing diagrams support that the nature of the aggregation is J-aggregation. The compound, 2, exhibits an irreversible mechanoluminescence (ML) property with a drastic colour change from blue to green (λmax, 445 nm → 512 nm) upon grinding. However, it undergoes a reversible transition with the same colour change (blue → green) through applying pressure axially (using a hydraulic press). The reversible transition is observed by lowering the temperature of 2 to that of liquid nitrogen. The causes of such transitions showing variations in the emission colour upon different triggers have been investigated. In addition, 2 has been successfully tested for the sensing of Zn(II) and shows a rare turn-on luminescence change, the mechanism behind which has been explored. The detection limit for Zn(II) is determined to be 0.064 ppm.


 Please wait while we load your content...
Please wait while we load your content...