Supramolecular hydrogen-bonded photodriven actuators based on an azobenzene-containing main-chain liquid crystalline poly(ester-amide)†
Abstract
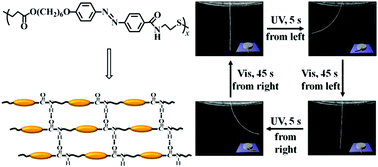
Development of physically crosslinked photodeformable main-chain liquid crystalline polymers (LCPs) is of significant importance (due to their good processability and recyclability, high thermal and mechanical properties, and outstanding shape changing effects) but remains a challenge. Herein, we report the facile and efficient synthesis of a new azobenzene (azo)-containing main-chain LCP with both amide and ester groups in its backbone (i.e., poly(ester-amide) or PEA) and photomobility of its supramolecular hydrogen-bonded fibers. The azo-containing PEA was readily obtained in a high yield by the first synthesis of an acrylate-type azo monomer with an N-hydroxysuccinimide carboxylate end-group and its subsequent Michael addition–amidation cooperative polymerization with cysteamine under mild conditions. The above polymerization mechanism and the exact chemical structure of the resulting azo polymer were elucidated by performing a model reaction. The obtained azo polymer exhibited high thermal stability, a low glass transition temperature, a broad range of smectic liquid crystalline phases, and reversible photoresponsive behavior, and its amide groups proved to form strong hydrogen-bonding interactions among polymer chains. Its supramolecular hydrogen-bonded fibers with a high alignment order of mesogens were easily prepared via the simple melt spinning method, and they showed much higher mechanical strength than many physically and chemically crosslinked photomobile side-chain LCP systems and more rapid photoinduced bending than both the previously reported physically crosslinked main-chain LCP and some typical chemically crosslinked side-chain LCP-based fibers even at ambient temperature. The decisive role of the hydrogen bonding-induced physically crosslinked networks in the photomobility of PEA fibers was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...