On the mechanical and electrical properties of self-assembly-based organosilicate porous films†
Abstract
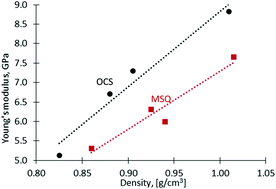
The effect of the replacement of Si–O–Si by Si–CH2–Si groups on the mechanical and electrical properties of silica-based hybrid sol–gel thin films is reported. For a reliable inference, two sets of organosilica films were synthesized – one consisting of a silica matrix decorated with methyl groups (Si–CH3) while the other further incorporating bridging methylene (Si–CH2–Si) functionalities. As a result, at the film density of 0.87 g cm−3, a higher Young's modulus of 6.6 GPa was deduced for the film containing Si–CH2–Si groups compared to 5.3 GPa for the one with Si–O–Si functionalities. Concurrently, the introduction of the methylene bridging groups leads to a dielectric constant increase from 2.12 to 2.27. Furthermore, the type of surfactant, ionic or nonionic, employed as a templating agent has a negligible effect on the electrical properties and the reliability performance of the porous organosilica films.


 Please wait while we load your content...
Please wait while we load your content...