Charge-transfer complexes of dinuclear ruthenium compounds/polyoxometalates for multistate electrochromism covering ultraviolet, visible, and near-infrared region†
Abstract
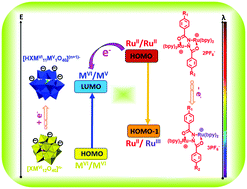
A series of novel charge-transfer electrostatic complexes between anionic polyoxometalates and cationic dinuclear ruthenium compounds have been prepared. An inter-molecular charge transfer from the electron-donor MCH-Ru to the electron-acceptor PMo12 occurs to generate two mixed-valence species of RuII/RuIII and MoVI/MoV in the complex; consequently, complementation of their corresponding charge-transfer absorption bands can obtain a full spectrum covering ultraviolet, visible, and near-infrared regions in its ground state with no need for external stimuli. Diverse polyoxometalates with various structures and charge densities were investigated and results show that both energy matching and molecular structures play important roles in the charge transfer process. Amphiphilic dinuclear ruthenium molecules enable their corresponding complexes to form supramolecular assemblies, morphologies of which also correlate to efficiency of the charge transfer. In addition, smart ternary memories were realized by using these complexes with three electrochemical inputs and three optical outputs. These hybrid complexes are promising for applications in multistate electrochromic materials and devices with tunable photoelectric properties.


 Please wait while we load your content...
Please wait while we load your content...