NIR mechanochromic behaviours of a tetracyanoethylene-bridged hexa-peri-hexabenzocoronene dimer and trimer through dissociation of C–C bonds†
Abstract
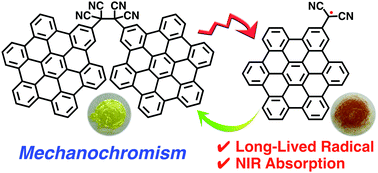
Oxidation of dicyanomethyl-substituted hexa-peri-hexabenzocoronenes (HBCs) furnished a tetracyanoethylene-bridged HBC dimer and trimer. The solid state structures of the oligomers were elucidated using single crystal X-ray diffraction analysis. In the solution state, these HBC oligomers exhibited conformational isomerism, depending on solvents and temperatures. Moreover, solid samples of the HBC dimer and trimer exhibited mechanochromism, showing near IR absorption upon grinding through generation of radical species. The formed radical was stable for 4 months, indicating the persistent nature of the radical species. These NIR mechanochromic behaviours of the HBC oligomers were originated from the reversible C–C bond dissociation and formation of a tetracyanoethylene linkage between the HBC units.


 Please wait while we load your content...
Please wait while we load your content...