Tuning of electronic properties via labile N→B-coordination in conjugated organoboranes†
Abstract
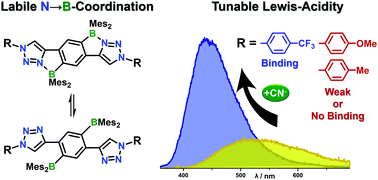
In this report we demonstrate that labile intramolecular N→B-Lewis pair formation can serve to tailor the properties of π-conjugated electronic materials. A series of boranes has been prepared by copper-catalyzed 1,3-dipolar azide–alkyne ‘click’-cycloaddition with a bifunctional borane (B2-H), which allowed the introduction of electronically varied aryl-substituents on the triazole ring (p-MeOPh, p-MePh, p-F3CPh), and also the preparation of a polymer (PTrz) with number-average molecular weight Mn = 7.2 kDa (PDI = 2.1, DPn ≈ 8.2). The optical and electrochemical properties of the boranes have been investigated in detail, and complemented by DFT calculations and anion binding studies. 11B NMR and dynamic 1H NMR show that the N→B-coordination persists in solution but is comparatively weak, and the boranes undergo rapid thermal exchange between closed, N→B-coordinated and open, non-coordinated conformers. UV-vis-absorption spectra exhibit only a minor impact of the derivatization, while fluorescence measurements show a direct correlation between the peripheral substituents and the observed Stokes shifts (3200 to 10 000 cm−1), fluorescence lifetimes (τ = 23–11 ns) and quantum yields (φ = 17.3–6.4%). Titration with cyanide shows that the strength of the N→B-bond can be incrementally tuned by variation of the peripheral substituents, to allow for direct binding of cyanide to boron.


 Please wait while we load your content...
Please wait while we load your content...