Insight into thin-film stacking modes of π-expanded quinoidal molecules on charge transport property via side-chain engineering†
Abstract
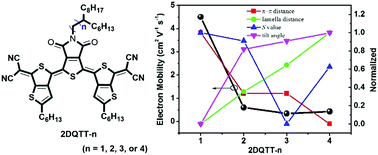
Control of molecular ordering and packing of π-conjugated molecules in the solid state is crucial for enhancing the charge transport properties in organic electronics. A series of quinoidal materials based on different alkyl-chain branching positions on the thieno[3,4-c]pyrrole-4,6-dione moiety flanked with unsymmetric thieno[3,4-b]thiophenes (2DQTT-n) are synthesized. By the combination of organic thin-film transistor performances and thin-film characterization, we clarified the influence of the branching position on the film microstructure/molecular packing and charge transport properties. Air-stable solution-processable n-channel 2DQTT-n derivatives show dramatic changes in film morphology and molecular packing, which leads to disparate electron mobilities ranging from ∼0.34 to 4.5 cm2 V−1 s−1. 2DQTT-1 with a branching point at the two-position in the alkyl side chain results in a 3D molecular packing with a lamellate morphology, and an electron mobility of up to 4.5 cm2 V−1 s−1 using an annealing temperature of just 80 °C. In contrast, the other three materials exhibit polymorphs and 2DQTT-3 and 2DQTT-4 even show mix-oriented crystallites which are highly disadvantageous to charge transport. These results demonstrate that variation of the alkyl-chain branching point is a powerful strategy to tune the stacking modes in the thin-film state, which enables high charge transport properties.


 Please wait while we load your content...
Please wait while we load your content...