Determining the diffusion mechanism for high aspect ratio ZnO nanowires electrodeposited into anodic aluminum oxide†
Abstract
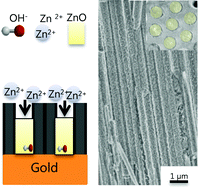
High aspect ratio ZnO nanowires have been uniformly grown into anodic aluminum oxide (AAO) templates by electrodeposition at a constant potential using peroxide solution. The influence of the ZnCl2 to H2O2 concentration ratio, reduction potential and electrodeposition temperature on the diffusion mechanism, filling ratio, morphology and crystallographic orientation of ZnO nanowires is studied. A correlation between the electrodeposition parameters, through the diffusion mechanism, and the morphological and structural properties of the ZnO nanowire arrays is presented. The diffusion coefficient was found to depend on the zinc chloride concentration, reduction potential and electrodeposition temperature for the electrodeposition of ZnO nanowires into AAO templates. The filling ratio was found to be influenced by the reduction potential and electrodeposition temperature. The morphology of ZnO nanowires is affected by the ZnCl2 concentration and reduction potential, but not by the deposition temperature. The crystallographic orientation of the ZnO nanowires depends on the reduction potential. These nanowires exhibit a high aspect ratio and uniform growth up to 50 μm thickness into AAO templates.


 Please wait while we load your content...
Please wait while we load your content...