Benzoindolic squaraine dyes with a large two-photon absorption cross-section†
Abstract
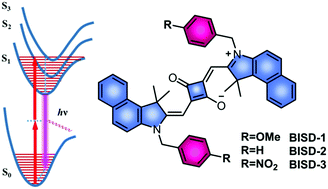
Near-infrared (NIR) emission and two-photon excited fluorescence (TPEF) are both desirable features for bioimaging because they offer several advantages, such as deep tissue penetration, high spatial resolution and low background noise. However, incorporation of NIR emission and TPEF into the same labeling dye molecule remains a formidable challenge as it requires three features simultaneously: large two-photon absorption cross-section (δ), high fluorescence quantum yield (Φ) and an appropriate NIR absorption/emission wavelength. Herein, we report a theory-assisted design of novel benzoindolic squaraine (BIS) dye molecules that exhibit a high-performance NIR emission and TPEF properties simultaneously. First, the planarity of the BIS core extended the π-framework, which leads to NIR emission at 682 nm with a quantum yield greater than 40%. Second, we utilized the local electric field effect by the addition of non-conjugated D/A moieties to the BIS core to modulate the two-photon absorption (TPA) cross-section (δ) values. Natural transition orbital calculations suggest that non-conjugated D or A groups do not affect the one-photon photophysical properties of BIS dyes, but can alter the molecular orbitals involved in the Sn ← S0 (n ≥ 2) TPA process. With this new strategy, we successfully obtained a methoxyl-modified molecule (BIS-1), which presents a TPA window between 780 and 950 nm, with the largest δ value above 12 000 GM.


 Please wait while we load your content...
Please wait while we load your content...