Hydrogen bonded capsules by layer-by-layer assembly of tannic acid and poly(2-n-propyl-2-oxazoline) for encapsulation and release of macromolecules†
Abstract
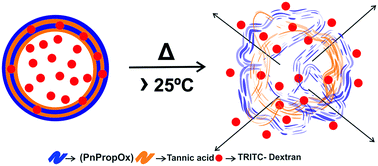
We report hydrogen bonded capsules with the built-in ability to release loaded bioactive molecules at a physiological temperature of 37 °C. The use of neutral and non-toxic building blocks such as tannic acid (TA) and poly(2-n-propyl-2-oxazoline)s (PnPropOx) as hydrogen bonding donor and acceptor results in stable hollow capsules. The temperature induced morphological changes of the shell were investigated using a scanning electron microscope and an optical microscope and revealed pore formation in the shell when the temperature (T) increases beyond the cloud point temperature (TCP) of PnPropOx. Furthermore, confocal laser scanning microscopic investigation of the hollow capsules loaded with different probes of varying hydrodynamic diameters revealed that the open and closed state of the capsules could be effectively manipulated by varying the incubation time and hydrodynamic radius of the probes. Such hydrogen bonded capsules have high potential for use in temperature responsive sustained drug delivery applications.


 Please wait while we load your content...
Please wait while we load your content...