HDAC inhibitor conjugated polymeric prodrug micelles for doxorubicin delivery†
Abstract
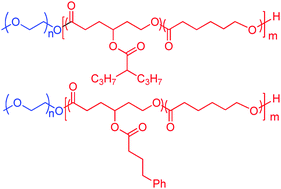
Amphiphilic diblock copolymers bearing histone deacetylase inhibitors (HDACis) (4-phenyl butyric acid and valproic acid) were synthesized by ring-opening polymerization of γ-4-phenylbutyrate-ε-caprolactone (PBACL), γ-valproate-ε-caprolactone (VPACL), and ε-caprolactone (CL) from a poly(ethylene glycol) (PEG) macroinitiator. These amphiphilic diblock copolymers self-assembled into stable pro-drug micelles and demonstrated excellent biocompatibility. High loading of doxorubicin (DOX) up to 5.1 wt% was achieved. Optimized micelles enabled sustained drug release in a concentration-dependent manner over time to expand the therapeutic window of cytotoxic small molecule drugs.


 Please wait while we load your content...
Please wait while we load your content...