Material design and photo-regulated hydrolytic degradation behavior of tissue engineering scaffolds fabricated via 3D fiber deposition†
Abstract
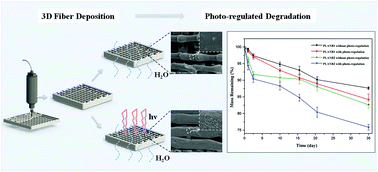
An ideal behavior of a tissue engineering scaffold is that it degrades and reshapes at a rate that matches the formation of new tissues. However, this ideal situation may not occur as the scaffold often undergoes too slow or too fast degradation. To test the promise of the active control of scaffold degradation, in this work, a photo/water dual-degradable porous scaffold was designed and fabricated using a 3D fiber deposition (3DF) system from a linear biopolymer (named PLANB) that combined the o-nitrobenzyl linkages and hydrolysable ester bone in the polymer chains. The chemical structure, molecular weight and polydispersity of PLANB were characterized by IR, NMR, GPC and MALDI-TOF-MS. The thermal properties of PLANB evaluated by DSC and TGA assays enabled a 3DF printing at the temperature around its melting point without chemical changes. The introduction of a real-time IR (RTIR) technique not only facilitated the determination of photolysis kinetics and quantum yield, but also enabled the capture of intermediate products during the photo-cleavage process of PLANB scaffolds. A minute-scale daily photolysis combined with a continuous hydrolysis process was implemented to test the photo-regulated hydrolytic degradation behavior of PLANB scaffolds in vitro, and the results obtained from both the SEM image and the mass loss profile demonstrated a porous-void microstructure along the strands of scaffolds and an apparent increase of the mass loss amount compared with the control group without photo-irradiation. Furthermore, PLANB scaffolds showed low cytotoxicity to L929 cells and performed well in promoting cell adhesion. It can therefore be concluded that such scaffolds have great potential in offering a diverse range of control over degradation kinetics of tissue engineering scaffolds to be tailored to individual tissue regeneration situations.
 Please wait while we load your content...
Please wait while we load your content...